Maintaining herd health and preventing disease is much more cost-effective than treating conditions. A well-designed herd health program minimizes death loss, introduction of new disease and loss of production efficiency due to disease. The best program for ensuring a high-quality health status for the herd on an individual farm will require the use and adaptation of an appropriate combination of the most current herd health techniques and technologies.
Risk management
Risk management plays a vital role in the development and implementation of an effective health program. Risk factors of a disease are those characteristics of the host, environment and pathogens (disease agents) that interact to contribute to the development of the disease. The risk management concept accounts for these complex interactions and serves as the foundation for the formulation and application of control measures (Figure 1).
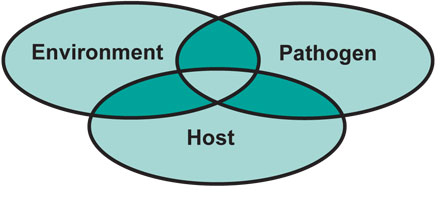 Figure 1. Risk management accounts for risk factors, which are interactions between the host, pathogens and the environment that contribute to development of a disease. Risk management also serves as the foundation for forming and applying control measures for the disease.
Figure 1. Risk management accounts for risk factors, which are interactions between the host, pathogens and the environment that contribute to development of a disease. Risk management also serves as the foundation for forming and applying control measures for the disease.
Environmental risk factors
The many environmental risk factors include sanitation, wind, rain, snow, ventilation and air quality, water quality, and population density. These risk factors can have both direct and indirect effects. Certain conditions allow more pathogens to survive in an environment, which increases contamination and raises the level of disease challenge to the animals in that environment. The effects of cold, heat, poor ventilation and crowding impose stress that weakens the animals’ immune response and increases the animals’ susceptibility to disease.
Pathogen risk factors
Pathogen risk factors include its virulence characteristics (ability to cause disease), reservoir of infection (source of the pathogen), amount of the organism to which the animal is exposed and mode of transmission. Typically, a pathogen causes a characteristic disease, but virulence may vary between strains of the disease agent with one strain causing a more severe level of disease. The ability of a pathogen to spread from animal to animal also can vary with strains. Vaccines may be effective against one particular strain of an agent but not against a different strain. Knowing the reservoir of infection or the source of the pathogen and mode of transmission can assist in formulating control measures.
Host risk factors
Host risk factors include the animal’s genetics, immune and nutritional status, and age at infection. Colostrum, the first milk a cow produces, provides antibodies against common diseases of newborn calves. The level of colostral immunity in a newborn calf is a major determinant of both resistance to and survival of these diseases.
Several intestinal tract diseases that cause diarrhea are common in newborns due to high susceptibility at a young age but are uncommon in older animals because susceptibility decreases with age. However, some level of disease challenge is always present. Figure 2 depicts the concept of disease resistance versus challenge. As long as the level of resistance is maintained above the level of challenge, disease is unlikely to occur (Figure 2A). When the resistance level drops below the challenge level, the risk of disease is high (Figure 2B). The risk of disease is also high when the challenge level rises above the resistance level (Figure 2C). When reduced resistance and increased challenge occur at the same time (Figure 2D), animals are at extreme risk and disease outbreaks are likely. The best possible situation is when the resistance level is raised while, at the same time, the challenge level is lowered (Figure 2E).
These guiding principles are used to develop, implement and monitor an effective health program. Work with your veterinarian to establish a vaccination schedule and management practices based on the prevalence and impact of specific diseases in your area. Unless a specific disease problem arises, minimum requirements should suffice. Primary consideration is given to infectious diseases that cause diarrhea, pneumonia, abortion, infertility and sudden death. Your veterinarian can help you determine the timing of initial and booster vaccinations that will maximize protection against the various diseases.
Also, establish internal and external parasite control, based on your area, for each of the production stages. Monitor the level of parasitism through routine fecal examination for internal parasites and clinical observation of animals for external parasites, and refine the program as needed. About six weeks after turnout onto pasture is when fecal egg count most closely matches worm burden (the number of worms in the animal).
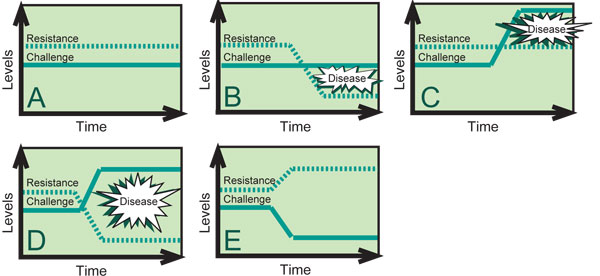
Figure 2. As long as the level of disease resistance is maintained above the level of disease challenge, disease is unlikely to occur.
Birth to weaning
Many management, environmental and physiological factors have a profound effect on the health of the young calf. During their first several months, calves are most susceptible to infectious diseases of the digestive and respiratory tracts. About 75 percent of the mortality of dairy animals less than 1 year old occurs during the first month of life.
Calf health begins with prepartum cow care. An adequate dry period (minimum of six weeks) is necessary for the cow to replenish her reserves and store sufficient levels of antibodies in colostrum to protect the calf. Dry-cow vaccinations provide adequate immunization and enhance colostral antibody levels. When choosing which vaccinations to give, consider any specific health problems present on the dairy. Also, adequate bunk space for eating and pen space for lying down are necessary to optimal cow health during dry-cow care. Be sure each cow has at least 30 inches of bunk space and 100 square feet of pen space.
Monitor cows during the calving process and help when needed. If maternity pens are used for calving, sanitation is the highest priority. Keep the maternity pens clean and dry. A pen should be used by one animal at a time and must be cleaned and disinfected between each use. Separate maternity pens from other pens and animals, and use them only for calving. Do not house sick animals in the maternity pens.
Outside lots used for calving should be well-drained and covered with grass and should provide protection from the weather, such as windbreaks and shade. Wet or muddy areas should be fenced to prevent cows from loitering or calving in these areas. To reduce manure buildup in the maternity lots, leave animals in them for the shortest possible time.
Dip the newborn calf’s navel cord in 7 percent iodine immediately after birth. As soon as possible after birth, separate the calf from the cow and place it in a clean, dry, draft-free environment. House calves individually in hutches at least 4 feet apart, or in groups in a well-drained pen. If the calves are housed in groups, each group should have no more than 8 to 10 animals of similar age for the first several weeks of age.
Calves should receive 10 percent of body weight of high-quality colostrum within the first 12 hours of life: about 1 gallon for Holstein calves and 3 quarts for Jersey calves. Ideally, the calf should receive 2 quarts of colostrum within 1 hour of birth and an additional 2 quarts within the next 6 to 8 hours. If this is not possible, give the calf the full amount at the initial feeding. A calf that receives the full amount may not want to drink at the next feeding 12 hours later. The producer should not force this feeding but wait another 12 hours, when a vast majority of calves readily drink.
Monitor colostrum quality to ensure that newborn calves are receiving high-quality colostrum. Antibody levels in colostrum from older cows are consistently higher than from first-lactation cows. A colostrometer is an excellent instrument for measuring colostrum quality. When using the colostrometer, make sure the colostrum is at room temperature.
Producers can maintain a frozen-colostrum bank with colostrum from mature cows in case high-quality colostrum is unavailable. However, several diseases can be transmitted in colostrum. The diseases of major concern are Johne’s (pronounced “Yo-Knees”) disease and bovine leukosis (BLV). Once an animal is infected with either of these diseases, it remains infected for life. There is no prevention and no treatment for these diseases. So, calves should not receive colostrum from cows known to be positive for Johne’s or BLV.
During the first month of life, the most common cause of sickness and death is calf scours (diarrhea). Several pathogens, including E. coli, rotavirus, coronavirus, salmonella, coccidia and cryptosporidia, cause calf scours. Although these agents can be present year-round, calf scours can become a significant problem under conditions of crowding, cold stress, inadequate nutrition and poor sanitation. Prevention is key. Adequate high-quality nutrition and clean, dry, draft-free housing are essential components of a prevention program. Specific preventive and treatment measures may be required in some situations.
Young calves tend to become ill and die more during cold, wet, windy weather. In a cold environment, calves’ maintenance energy requirements are much higher, so their diet should be adapted. The dietary changes can include increasing fat content of the milk replacer or adding another feeding to the daily schedule. Calves fed milk replacers with less than 20 percent fat are at greater risk during the winter months, if housed in a cold environment. Young calves must be monitored on a regular basis, and the amount of liquid diet should be altered to sustain energy levels during cold conditions. Young calves should be offered a balanced calf starter and water as early as 1 week of age to increase the nutrient density of the total diet without inducing milk scours.
Two important health-management practices that should be implemented during the preweaning period are dehorning and removal of extra teats. Calves can be dehorned as early as 2 to 3 weeks of age or as soon as the horn button can be felt. To prevent injury to the calf or the operator, use a restraint device such as a calf cradle or table; local anesthetics and/or analgesics are highly recommended. Electric dehorning is a safe, humane method for dehorning young calves. Proper procedure calls for the surface of the dehorner to be “cherry red” before it is touched to the horn button. The dehorner should be applied to the horn button using steady pressure for about 10 to 15 seconds or until a continuous copper-colored ring is displayed around the base of the horn. This procedure causes minimal pain to the calf and provides rapid destruction of the horn button. Each time a calf is restrained, examine the udder for extra teats. The four regular teats should be symmetrically arranged. Extra teats are usually smaller in size and located close to the main teats. Snip off the extra teats with a pair of scissors, and paint the cut surface with a topical antiseptic. If you are not absolutely sure which teats are the extras, consult your veterinarian.
Vaccinations usually are unnecessary in the preweaning stage if the calf has received adequate colostral protection and is kept in a healthy environment. The calf’s immune system does not respond to stimulation, from either disease exposure or vaccination, to the same degree as an adult animal until after 3 months of age. Also, colostral antibodies can interact with vaccines and render most of them ineffective until the calf is more than 3 months old. Specific situations may require certain vaccinations in the preweaning stage. In this case, an intranasal or modified live vaccine (MLV) is preferable. Any vaccine given preweaning should be boostered after the calf reaches 3 months of age.
Table 1. Recommended housing specifications for heifers.
| Age group | Maximum head per group | Age spread in group | Area/head | Feed-bunk space length/head |
|---|---|---|---|---|
| 0 to 2 months | 1 | — | 24.0 sq ft | — |
| 2 to 3 months | 5 | 1 week | 28.0 sq ft | 20 inches |
| 3 to 4 months | 10 | 2 weeks | 28.0 sq ft | 20 inches |
| 4 to 6 months | 20 | 3 to 4 weeks | 37.5 sq ft | 27 inches |
2 to 6 months
Colostral immunity begins to decline between 2 and 6 months of age, and the young calf begins to produce its own immunity. During this transition, calves can go through a period when they are more susceptible to diseases.
Weaning often occurs in this transition period. Weaning is one of the most stressful periods in the life of the animal. Given the increased level of stress, waning of colostral protection and increased animal contact due to grouping, take special care during the weaning process to reduce the likelihood of disease outbreaks.
Calves should remain in the hutches for two weeks after weaning to promote grain intake and decrease the stress of weaning. Calves can then be moved from the hutches into small groups. Small groups ease the transition from individual housing to group competition. These practices decrease the stress of weaning and help the calves maintain weight gains.
Calves should receive their first series of vaccinations or boosters to any vaccines they were given earlier. Refer to the “Health management checklist: 2 to 6 months” for timing of vaccinations. Many vaccines are available to protect dairy replacement animals from diseases. Vaccines commonly used provide protection from brucellosis, infectious bovine rhinotracheitis (IBR), parainfluenza (PI3), bovine virus diarrhea (BVD), bovine respiratory syncytial virus (BRSV), clostridia (blackleg) and leptospirosis. Vaccines also are available to provide protection against pasteurella, hemophilus and pinkeye. Discuss the specific needs of your farm with the herd veterinarian. When administering vaccines, follow the label instructions.
Respiratory diseases are the most common health problem in this growth stage. Prevention starts with proper housing and nutrition (energy is of utmost importance). Calves reared in confinement require clean, dry, properly ventilated housing. Provide clean, fresh water and adequate feed-bunk space. Group calves by size and age. Table 1 lists recommended housing specifications.
Pasture-rearing calves from weaning to 6 months of age can be successful provided the quantity and quality of available grass is carefully monitored. Pasture alone is generally not recommended for calves less than 4 months old because the composition of pasture often fails to supply adequate nutrients. Calves 4 to 6 months old can graze on pasture with grain supplementation. The amount and blend of grain fed will depend upon the age of the animals and should be balanced to complement the quality of forage being fed.
Gastrointestinal parasites can be a serious problem affecting the growth and performance of dairy calves reared on pasture. The eggs of gastrointestinal worms are passed onto the pasture in the feces of infected animals. The eggs hatch in the fecal pats, where the larval form of the worm grows to an infective stage. These infective larvae then move to blades of grass and then are ingested by the animal to complete the life cycle.
Parasite control requires the establishment of good parasite management practices to reduce parasite loads in the animal and minimize pasture contamination. Calves less than 1 year old have little or no resistance to internal parasites, so calves should begin a routine deworming program starting at weaning.
A deworming program should address the inhibited larval form of Ostertagia (brown stomach worm). The numbers of inhibited larval forms increase during adverse weather conditions such as dry, hot summers and cold winters. For adequate control, appropriate timing and use of deworming products that are effective against the inhibited larval forms are necessary. Both the summer- and winter-inhibited forms are found in Missouri. The major inhibited form will depend on the farm’s location in the state. Consult your veterinarian to find out the inhibited form most common in your area.
Strategic deworming involving the use of anthelmintics (dewormers) at selected times combined with various systems of grazing management has been successful. The “treat and move” system involves deworming in late spring before infection pressure is high and then moving the animals to pasture ungrazed by cattle since the previous autumn. In rotational grazing systems, deworming at the start of the rotational pattern effectively “cleans out” the animals so that paddock contamination is reduced. In rotational systems where multiple groups of animals follow one another through the rotation, younger calves should lead, followed by older animals. At the end of the grazing period, pastures can be “dragged” to break up fecal pats and expose eggs and larvae to the effects of drying and sunlight, which aid in reducing contamination.
Coccidiosis is another internal parasitic infection that can cause losses in calves. Coccidia are single-cell protozoa that live within the cells of the digestive tract. These organisms can cause extensive damage to the intestinal tract of calves 2 to 12 months of age. Severely infected calves may show signs of bloody diarrhea. Coccidiosis at the subclinical level (undetectable by usual clinical observations) reduces the growth rate of calves. Feeding concentrates, supplements and milk replacer that contain a coccidiostat will help prevent coccidiosis and promote healthy weight gains.
Fly control is required during the fly season. It should begin before the fly season to prevent a population buildup and should continue until the first hard freeze. Routine manure and bedding removal help to eliminate fly larval development. The use of sprays on both the premises and livestock provides additional control. Insecticide ear tags and dust bags, along with systemic products, may be included in a fly control program.
Animals housed in winter are susceptible to lice infestations. In young calves, severe lice infestations can lead to poor performance and anemia. Control measures for lice include a variety of sprays, dusts and pour-ons. Closely monitor animals for signs of lice, then institute appropriate control measures.
6 to 24 months
Between the ages of 6 and 24 months, the major health problems of heifers on pasture are internal parasites, external parasites and infectious diseases that can cause respiratory and reproductive problems. Maintain internal and external parasite control as described in the previous section.
Administer booster vaccinations against respiratory and reproductive diseases (IBR, PI3, BVD, BRSV and leptospirosis). Ideally, these boosters should be given between 60 and 30 days before breeding during a prebreeding examination. If bulls are to be turned in with the cows, be sure they are vaccinated as well. When administering vaccines, follow label instructions under advisement of your veterinarian.
The breeding program for replacement heifers is a critical part of a successful dairy operation. Calving heifers by 24 months of age offers several economic advantages. Composite results from studies over the years show lifetime milk yield and lifetime profit are maximized when heifers calve for the first time between 23 and 25 months of age. Increasing age at first calving increases the nonproductive life of the heifer and delays returns of income from milk sales. It also increases the inventory of replacement heifers needed to maintain herd replacement rates. In seasonal breeding programs, replacement heifers must be developed so that their breeding season matches that of the lactating herd.
To calve by 24 months of age, a heifer must conceive by 15 months of age. Age and, more important, size are the major factors contributing to the onset of puberty in heifers. Developing heifers to the proper target weight and height by 14 months of age ensures a higher percentage of cycling heifers at the time of breeding. Growth charts for various dairy breeds have been developed that can be used to monitor the progress of replacement heifers and recognize problems in feeding and management while there is still time to take corrective action. In other breeds, a target weight of 65 percent of mature weight at first breeding can be used as a guideline to monitor replacement-heifer development.
The decision to use artificial insemination, natural service or a combination of the two needs careful consideration. This decision should be based on the availability of labor and breeding management skills. Before deciding to breed heifers using natural service, explore the disadvantages. Bulls often are difficult to handle, can become dangerous and will increase wear and tear on facilities. They may be subfertile or infertile and can carry venereal diseases. Alternatively, artificial insemination (AI) programs require additional labor and management. A successful AI program requires heifers that are properly developed and cycling, adequate and accurate heat detection and appropriate insemination techniques. Estrus synchronization used in conjunction with AI can improve labor efficiency on most operations.
Culling heifers is one of the most difficult and costly decisions facing the dairy producer. Make this decision in a timely manner, however, because delayed culling will increase expenses and lower returns. Respiratory diseases often are the major culprits that result in slowed growth and “poor doers.” Failing to cull these heifers will result in delayed age at breeding, potential difficulty in calving and poor cow performance.
The most common physical abnormalities encountered in heifers are freemartinism and white heifer disease. Freemartinism occurs when a heifer is born as a twin with a bull. Developmentally, the male gonads develop before the female reproductive organs. Because of the shared blood supply, the male hormone prevents the proper development of the female reproductive organs.
White heifer disease is a genetic disorder that also results in failure of the female reproductive organs to develop. Both conditions produce heifers that lack portions of their reproductive tracts and are infertile. Perform thorough rectal exams when heifers are between 6 and 12 months of age to evaluate the internal reproductive organs and identify these problems.
Various injuries and disease processes can render a heifer unfit for the rigors of milk production. These injuries include conformational problems with feet, legs and the mammary gland. Upon diagnosis, heifers should be culled to minimize production costs.
Adult herd
An adult-herd health program focuses mainly on maintaining adequate herd immunity through vaccination and minimizing the effects of metabolic diseases, mastitis and reproductive disorders. As with the young stock, establish health procedures based on the prevalence and impact of specific diseases. Fulfill minimum requirements unless a specific disease problem arises. Monitor the incidence of health problems and refine the program as needed.
Vaccination schedules in adult dairy herds typically follow one of several protocols. The most common protocol involves administering vaccines either at dry-off or during the dry period, or at both times. An advantage of this protocol is that the animals are in the same stage of production, so booster vaccinations at this time increase the resistance to diseases that may affect reproductive performance in the subsequent pregnancy. Vaccinating at this time also increases the level of immunoglobulins in colostrum, which increases potential protection for newborn calves. A major disadvantage is the challenge of ensuring that all animals receive the health protocol. Cows can be missed unless good records are kept and the protocol is strictly adhered to. Over time, herd immunity decreases, which makes the animals more susceptible to disease challenge.
Some producers simply schedule complete herd vaccinations on a specific day. The advantage to this protocol is that all animals are vaccinated. The disadvantage is that the animals are in different stages of production, so increased resistance may not match the disease challenge. Also, a drop in milk production can be expected for a period of several days from the stress of working the animals and the effects of the vaccinations.
Vaccinations common to all dairies include infectious bovine rhinotracheitis (IBR), para-influenza (PI3), bovine virus diarrhea (BVD), bovine respiratory syncytial virus (BRSV) and leptospirosis. These vaccines should be administered on at least an annual basis, preferably prior to breeding. Vaccines also are available for a variety of other health problems. For example, scours vaccines are administered to cows to provide mastitis control and colostral protection for calves. The timing of administration is critical to the success of these vaccines. Discuss the specific vaccine needs of your farm with the herd veterinarian. When administering vaccines, follow the label instructions under the advisement of your veterinarian.
Metabolic diseases
Metabolic diseases are defined as disorders that are nutritional in origin and often result in acute symptoms that require treatment. Metabolic disorders most often occur from just before calving through peak lactation. The increased susceptibility during this time is associated with changes in metabolism during the transition from the relatively small nutritional demands of the dry period to large nutritional demands with the onset of lactation. Primary threats are milk fever, ketosis and indigestion (lactic acidosis), but grazing herds also need to be protected from grass tetany and bloat.
Milk fever occurs at or near calving and is characterized by low blood calcium levels and muscular weakness. Besides the typical “downer cow” presentation, muscular weakness predisposes the cow to several calving-related complications, including prolonged calving, uterine prolapse and retained fetal membranes (afterbirth). Prevention and control centers around restricting calcium intake during the dry period so that calcium mobilization mechanisms in the body remain functional to move calcium from body stores at calving. Keeping calcium intake to less than 20 grams a day is ideal, but formulating diets to meet this requirement is very difficult. Diets with daily calcium intake below 80 to 100 grams a day are successful in minimizing the incidence of milk fever. Such a diet precludes the feeding of minerals containing calcium and feedstuffs known to be high in calcium, such as legumes, during the dry period. Use of anionic salts can help mitigate the effects of high potassium and/or calcium levels in the dry cow diet. Work with your nutritionist if you choose to use these feed additives.
Ketosis results from impairment in metabolism of carbohydrates and volatile fatty acids (VFA) that leads to hypoglycemia (low blood sugar). Body fat is mobilized in response to hypoglycemia, and incomplete fat metabolism leads to ketone formation (ketosis). Ketosis is most common four to six weeks after calving when the drain on the cow’s glucose reserves is highest. Thus under- or overconditioned cows with inadequate feed intake following calving are likely candidates. Any condition that leads to reduced or inadequate feed intake ultimately results in ketosis. The use of monensin has proven to decrease the number of clinical and subclinical cases of ketosis in the dairy cow.
Indigestion is associated with feed changes such as a switch from concentrate feeds to lush pasture or vice versa. Abrupt dietary changes or consumption of soured feedstuffs alters rumen pH and fermentation, which trigger indigestion. Depending on the abruptness of the change and the volume of feedstuffs consumed, this condition can vary from mild, simple indigestion to the more severe form (lactic acidosis), which can be life threatening. Indigestion can be a common problem in lactating cows when they are fed concentrates in the parlor during milking and then turned to roughages or pasture. This process, often called “slug feeding,” leads to continual fluctuation in ruminal pH and indigestion. The best prevention is feeding-management strategies that make dietary changes gradually. Major dietary transitions, such as switching dry-cow to lactating-cow rations, require an adaptation period of about three weeks. Gradual change from high-roughage to high-concentrate diets allows ruminal bacterial populations to adapt. Feeding rumen buffers such as sodium bicarbonate helps to reduce the risk.
Grass tetany, which is potentially fatal, is characterized by low blood magnesium levels and, often, low blood calcium levels. The most common occurrence is in lactating cows shortly after they begin grazing lush, succulent grass pasture. Low levels of magnesium in lush plants and/or the competition for absorption between magnesium and other minerals are thought to cause grass tetany. Heavily fertilized pastures, especially wheat, appear to increase the risk. Dietary supplementation with a magnesium source such as magnesium oxide for cattle on tetany-type pastures is used as a preventive.
Bloat is the excessive accumulation of fermentation gases within the rumen and can be in the form of free gas or foam (froth). Frothy bloat occurs in animals consuming a variety of feedstuffs. Lush forages have the potential to cause frothy bloat. The worst offenders include legumes such as alfalfa, sweet clover and red clover as well as lush wheat or ryegrass. Grazing management is important for control of bloat on pastures. Cattle should not be exposed to bloat-causing forages when hungry. Supplement with coarse roughages or grains to reduce intake of the pasture forages. Animals should be turned in to pastures after the dew has evaporated. Limit time on the pastures to less than two hours a day. On severely bloat-prone pastures, add the surfactant poloxalene (for example, Bloat Guard), which is highly effective in reducing losses from bloat.
Mastitis
Mastitis is an inflammation of the mammary gland that results from the invasion of pathogenic organisms through the teat streak canal. Mastitis is reported as the most common disease affecting dairy cows worldwide. The National Institute for Research in Dairying (NIRD) and the National Mastitis Council (NMC) have outlined and promoted detailed mastitis control strategies.
The major pathogens that cause mastitis are classified as contagious or environmental. Contagious pathogens are spread directly from animal to animal, and transmission of these agents occurs during milking. The most common contagious pathogens are Streptococcus agalactiae (Strep. ag.), Staphylococcus aureus (Staph. aureus) and Mycoplasma. Mycoplasma is very difficult to impossible to treat and most cows found with the organism need to be culled. If you suspect Mycoplasma, alert the lab you use for culturing because special media and conditions are needed to grow the organism, and the organism takes longer (10 to 14 days) to grow than the common bacteria. Environmental pathogens, as the name indicates, are commonly found in the environment. They are transmitted from the environment to the animal and can be spread at any time, not just during milking. The major environmental pathogens include E. coli (coliform), Streptococcus uberis (Strep. uberis) and Streptococcus dysgalactiae (Strep. dysgalactiae).
Keeping cows from being exposed to mastitis-causing organisms is impossible. Two major objectives of a mastitis control program are to prevent new infections and to reduce the duration of infections. To accomplish these objectives, many producers have adopted some form of the “five-point plan.” The five-point plan consists of
- Developing a proper milking procedure that considers hygiene,
- Performing teat dipping and disinfecting at milking,
- Maintaining milking machines,
- Using dry-cow therapy and treating clinical cases
- Culling persistently infected cows from the herd.
Milking procedure
Proper milking procedure includes premilking udder hygiene, stimulation of milk letdown, efficient milk removal and postmilking teat disinfection. The following principles are important for controlling the spread of contagious pathogens and preventing new infections caused by environmental organisms:
- Establish a regular milking schedule in a stress-free environment. Rough handling, loud noises and inconsistent milking intervals induce stress that can counteract the effects of oxytocin and milk letdown.
- Check udder and foremilk for mastitis. Look for early signs of mastitis, such as pain, heat or swelling.
- Ensure that teats are clean and dry before milking. If udders are excessively dirty, wash and thoroughly dry the udder and teats. Avoid excessive wetting of the udder. Use single-service towels for each cow. Wear sanitized latex gloves during milking. Many producers implement a predip milking procedure to clean and sanitize teats.
- Minimize the amount of air drawn into the system during attachment to minimize vacuum fluctuation. Adjust attachment during milking to keep the unit straight and level.
- Do not remove the milking unit under vacuum. As soon as the cow is milked out, shut off the vacuum and allow the teat cups to gently fall off the teats.
- Use an effective and safe postmilking teat germicide after every milking. Apply the germicide by spraying or dipping, and cover at least the lower half of the teat. Dry teats before exposing to cold and windy conditions. Maintain clean teat-dipping utensils.
- Milk current clinical cases and cows with high somatic cell counts (SCC) and chronic clinical mastitis last.
- Sample all clinical cases of mastitis. Take a sample from the affected quarter; label the vial with the date, cow number and quarter affected; and put the sample in the freezer or, if culturing for Mycoplasma, the refrigerator. If mastitis cases increase, the samples can be quickly sent off to be cultured.
Proper installation, function and maintenance of milking equipment plays an integral role in milking efficiency and has a direct influence on the status of udder health. Guidelines are listed in Chapter 11, Facilities Design, in MU Extension publication M168, Dairy Grazing Manual.
Dry-cow management
The proper management of dry cows is an extremely important component of a mastitis control program. The dry period offers the opportunity to improve udder health while the cow is not lactating. However, the beginning and end of the dry period are times of increased risk of infection. Cessation of hygienic milking practices such as teat dipping allows bacterial populations on teat skin to increase. Studies show that the teat canal is more penetrable early in the dry period and again late during the prepartum period. Marked changes in mammary gland secretions and in concentrations of protective factors during the dry period influence the variation in susceptibility to both environmental and contagious pathogens.
Dry-cow therapy is the infusion of long-acting antibiotic preparations into each quarter at dry-off and is an extremely important procedure in a mastitis control program. The use of effective dry-cow products reportedly eliminates 70 to 90 percent of existing infections and reduces the incidence of new infections by 50 to 75 percent. To prevent contaminating the udder and causing infections, maintain strict sanitation during the procedure. Follow these steps to perform dry-cow treatment:
- Completely milk out the udder.
- Dip or spray all teats with effective germicide.
- Disinfect each teat end with individual alcohol-soaked swabs. Swab far teats first and near teats last. Be careful not to touch teat ends after swabbing.
- Infuse each quarter with a single-dose syringe of dry-cow preparation. Use the partial insertion technique of syringe cannula (no more than 4 mm). Infuse near teats first and far teats last.
- A teat sealant may be used after the antibiotic treatment. Research has shown that for up to 10 to 14 days after dry off, as many as 50 percent of teats may not have fully keratinized, increasing the potential for infection by environmental organisms. If a herd has struggled with high bulk tank somatic cell count (SCC) or cows will be placed in a sacrifice paddock, the use of an internal teat sealant may be advisable.
- Dip or spray all teats with effective teat germicide immediately after treatment.
Follow all label directions, and observe milk withdrawal times, which are typically 30 to 42 days. Keep records of treatment dates to prevent potential residues in the subsequent lactation.
Therapy during lactation
Appropriate therapy of mastitis during lactation is another important component of udder health management. With the herd veterinarian, develop a treatment protocol for lactating cows. The level of udder health status and severity of cases are important considerations. The treatment protocol should be well defined and understood by everyone involved. Treatment should consider case characteristics, knowledge of the causative agent, stage of lactation, age of the cow, and duration and severity of the infection.
Culling chronic infections
The final step of the five-point plan is the selective removal from the herd of cows with chronic udder infections. Culling is the major control procedure for some specific mastitis pathogens, most notably infections with Staph. aureus. Cows infected with this organism have a poor cure rate (15 percent) and serve as a chronic source of infection for the rest of the herd. Most producers interpret this recommendation to mean that cows with recurrent episodes of mastitis should be eliminated. Certain strategies have established that cows having three or more cases of mastitis in a particular lactation be culled. Other strategies for deciding which cows with clinical cases of mastitis to cull involve evaluating other production parameters, such as age, feet and leg condition, milk production and stage of lactation. Available monitoring techniques and establishment of a defined culling program give producers a valuable opportunity to improve udder health by culling.
Abortion
Abortion causes significant economic losses for producers because of extended calving intervals, reduced milk production and loss of calves. Microorganisms that survive in the bloodstream of the cow and cross into the uterus can cause placentitis, or fetal death, and can cause abortion. Abortion events that occur only occasionally and at random are classified as sporadic. Abortion events that result in a high percentage of animals aborting in a short period of time are classified as epizootic. Microorganisms that are common in the environment of cows, such as Actinomyces pyogenes, Escherichia coli, Aspergillus, Bacillus and Streptococcus species, tend to cause sporadic abortion. Thus, reducing the number of microorganisms to which cows are exposed, through a prevention program focused on sanitation, will reduce the risk of sporadic abortion. Enhancing the health status of the cow through proper nutrition and environmental stress management (reduction of heat stress) will also reduce the risk of sporadic abortion.
Commonly recognized diseases include Brucellosis, Leptospirosis, Infectious Bovine Rhinotracheitis (IBR) and Bovine Virus Diarrhea (BVD). Prevention of epizootic abortion focuses on proper immunization and disease control measures aimed at the elimination of carrier animals through testing and culling. Consult your veterinarian for more detailed information on disease controls for epizootic abortion.
In addition, persistently infected BVD (BVD-PI) has become much better understood and needs to be addressed. (The most common way of testing for BVD-PI is submitting an ear notch from the calf. If BVD-PI is suspected, all calves, including bulls, stillborns, abortions and heifers must be tested. In addition, all newly purchased animals need to be tested.)
A less commonly recognized microorganism that can produce sporadic and/or epizootic abortion is Listeria monocytogenes, which is often associated with feeding moldy silage. The newest recognized cause of abortion, discovered in 1984, is Neospora caninum. Neospora is a protozoal organism that infects dogs and is passed in their feces, thus exposing cattle to the organism. A vaccine for prevention of this disease is available, but its effectiveness has not yet been determined. Eliminate cow contact with dog feces.
The risk of abortion can be reduced significantly through implementation of control measures that address environmental management aimed at reducing stress; proper nutrition and immunization to maximize immunity; and biosecurity measures focused on sanitation and the prevention of disease entry through new herd additions.
Lameness
Bovine lameness is a multifactorial problem that alters the animal’s gait and reduces performance. Many factors, including nutrition, genetics, facilities, environment and infectious organisms, predispose cattle to lameness. Two of the more commonly observed conditions on today’s dairy farms are laminitis (founder) and digital dermatitis (hairy heel warts).
Laminitis, or founder, is a disease condition of the claw. This condition begins with a disturbance in the microcirculation of the foot that leads to inflammatory changes at the hoof soft tissue junction and results in impaired horn production and hemorrhage in the sole and hoof walls, which causes double soles, sole ulcers and abscesses.
For diagnostic purposes, laminitis fits into one of three classifications:
- Subclinical
No immediate signs of lameness - Acute
Rapid onset of lameness - Chronic
Characteristic deformation of the claw that develops over time
The classical cause of laminitis is associated with feeding high levels of carbohydrates (ground grains), which results in rapid fermentation. The faster fermentation increases the level of lactic acid in the rumen, which triggers endotoxin release along with histamine response. All of this results in disturbance in the microcirculation of the foot and produces laminitis.
Endotoxemia, which often follows severe mastitis and metritis, is also associated with laminitis. The circumstances that lead to endotoxemia are often observed as acute conditions resulting in severe lameness.
Grass founder is a chronic form of laminitis associated with dramatic changes in nutrition, such as from poorly palatable stored winter forage to highly palatable lush spring pasture. Spring pasture is high in protein and soluble carbohydrates, both of which are considered factors that contribute to laminitis.
Digital dermatitis, or hairy heel warts, is a contagious superficial inflammation of the heel. Two types of lesions have been observed with this disease. In some cases, the lesions are proliferative with wartlike projections; in other cases, they are more erosive with an ulcerlike appearance. The definitive cause of digital dermatitis has not yet been determined. However, the most reasonable explanation is that dermititis is a multifactorial disease in which spirochetes, together with either bacteria or viruses, develop an opportunistic pattern creating the disease. Foot hygiene is critical in dermatitis prevention and control because cows with clean feet are less likely to contract digital dermatitis. Also, reduction of high-moisture conditions is mandatory. This disease is contagious and can be spread from infected cattle to noninfected cattle, so take extreme care when purchasing new herd additions and ensure that hoof-trimming equipment is sterilized between farms and cows. For treatment recommendations, consult your veterinarian.
Biosecurity
Biosecurity refers to management practices that reduce the chances of infectious diseases being introduced onto the farm by animals or people. It also refers to practices that reduce the spread of diseases found on the farm.
Certain segments of the livestock industry, most notably poultry and swine, have long-established, strict biosecurity plans. Although biosecurity measures are commonly discussed in general terms in the dairy industry, efforts to establish a coordinated biosecurity plan have recently received renewed interest. Establishing an effective biosecurity plan involves the following steps:
- Set current and long-term goals.
- Assess the risks of introduction or spread of the diseases in question.
- Define objectives for control or prevention of the diseases in question.
- Outline strategies to meet the defined objectives.
- Monitor progress.
Assessing the risks relates to the potential level of challenge to the herd. The level of relative herd risk can be classified on a herd basis as a closed, modified-open or open herd. In closed herds, all animals are raised on the premises, with no outside additions. These herds are located in areas of relative isolation with minimal contact with outside animals. Modified-open herds have small numbers of herd additions, such as the purchase of herd bulls or replacement heifers, on an intermittent basis. These herds are located in areas of moderate isolation with potential contact with outside animals from surrounding herds. Open herds have large numbers of outside animals introduced routinely. Open herds are often seen during the start-up phase of an operation, during a herd expansion or in herds with excessively high cull rates.
The concept of Hazard Analysis and Critical Control Points (HAACCP) has been used to establish objectives and define strategies in biosecurity plans. HAACCP requires an understanding of disease transmission and identifies specific points at which producers should intervene. Certain strategies are common and often relate to simple, good husbandry practices. Many of these practices have been described in this guide.
Reduce the risk of disease transmission on your farm by taking these precautions:
- Maintain clean, dry, sanitary environments.
- Prevent contamination such as manure on hair coat, feed and water.
- Separate animals younger than 1 year of age from the adult herd.
- Always work from younger to older animals.
- Do not use “waste” feed from older animals to feed younger animals.
- Wear clean boots and clothing, especially when working with calves.
- Isolate sick animals.
- Perform a necropsy on all dead animals.
- Dispose of dead animals promptly.
Prevent potential introduction of diseases from outside sources by taking these precautions:
- Admit animals only from herds with known good health status.
- Avoid buying animals from mixed sources.
- Isolate new animals for at least 30 days.
- Screen new animals for disease during an isolation period.
- Transport purchased cattle in farm-owned vehicles if possible. Hired transporters should start with a clean truck.
- Limit access to facilities by outsiders. Post warning signs.
Johne’s disease and BLV
Certain diseases, such as Johne’s disease and bovine leukosis (BLV), require special biosecurity considerations. As stated earlier, Johne’s disease and BLV do not have effective preventive measures such as vaccines, and once an animal is infected, it remains infected for life.
Johne’s disease is a bacterial infection that affects the lower gut and causes chronic diarrhea and wasting. Young animals from birth to 6 months are most susceptible to infection. However, the disease has a long incubation period that can last years, and animals do not typically show clinical signs until they are adults. About 1 to 5 percent of infected animals show clinical signs in a given year. As the disease progresses, the organism is shed in the feces in increasing numbers. The organism has been shown to survive in the environment for up to a year.
The major mode of transmission of Johne’s disease is fecal contamination from the infected animal. Colostrum and unpasteurized milk, especially from cows in the later stages of the disease, also can harbor the organism and serve as a source of infection. The disease may also be transmitted in utero (from an infected cow to its fetus); therefore, a calf can be born infected.
Fecal culturing and serologic blood tests are available for diagnosing Johne’s disease. However, these tests are not foolproof and require careful interpretation.
The following list describes major control measures for Johne’s disease:
- Prevent infections by closing the herd to animals with unknown Johne’s disease status.
- Secure replacements and herd additions from low-risk herds.
- Reduce infections by manure management.
- Reduce infections by colostrum and milk management.
- Reduce infections by management of infected animals.
Bovine leukosis (BLV) is a viral infection that affects lymphoid tissues, usually in the gut, uterus, heart, eye, spinal column and lymph nodes. The clinical signs of BLV relate to the tissues and organs affected. Like Johne’s disease, BLV has a long incubation period that can last years. Each year, 1 to 5 percent of infected animals show clinical disease. Clinical cases of BLV are most commonly seen in animals 4 to 8 years of age.
BLV is most often transmitted by the transfer of infected lymphocytes (white blood cells) from an infected animal. Bloodsucking insects, multiple-use needles, rectal palpation using a common plastic sleeve and surgical procedures such as dehorning can spread the disease. As with Johne’s disease, BLV can be transmitted in colostrum and milk, and in utero transmission has been reported.
Serologic blood tests are available for BLV. As with those for Johne’s disease, these tests require careful interpretation.
The following list describes major control measures for BLV:
- Prevent infections by single use of instruments and supplies such as sleeves and needles.
- Reduce infections by colostrum and milk management.
- Secure herd additions from known low-risk sources.
- Reduce infections by isolating and segregating infected animals.
- Reduce infections by testing and then culling/slaughtering infected animals.
- Reduce infections with insect vector control.
Growing interests and concerns about Johne’s disease and BLV has led many states, including Missouri, to adopt uniform guidelines for voluntary control programs. Information on these programs is available through your veterinarian.
References
- Bailey, K., M. Bennett, J. Garrett, D. Hardin, J. Hoehne, R. Randle, J. Spain, B. Steevens, and J. Zulovich, eds. 1993. Missouri dairy plan. Columbia, Mo.: University of Missouri Extension.
- Bailey, K., J. Garrett, D. Hardin, J. Hoehne, R. Randle, J. Spain, B. Steevens, and J. Zulovich, eds. 1994. Missouri system of dairy heifer production. Columbia, Mo.: University of Missouri Extension.
- Greenough, Paul R., and A. David Weaver, eds. 1997. Lameness in cattle, 3rd ed. Philadelphia, Pa.: W.B. Saunders.
- Howard, J.L., and R.A. Smith, eds. 1999. Current veterinary therapy 4: Food animal practice. Philadelphia, Pa.: W.B. Saunders.
- Radostits, O.M., K.E. Leslie, and J. Fetrow, eds. 1994. Herd health: Food animal production medicine, 2nd ed. Philadelphia, Pa.: W.B. Saunders.
- Van Horn, H.H., and C.J. Wilcox. 1992. Large dairy herd management. Champaign, Ill.: American Dairy Science Association.
- Youngquist, Robert S., ed. 1997. Current therapy in large animal theriogenology. Philadelphia, Pa.: W.B. Saunders.