Although nitrogen is quite stable in the atmosphere, the form of nitrogen in the soil can change very easily. Some forms of nitrogen can be changed to ammonia gas (NH3). Ammonia gas can be lost from the soil and return to the atmosphere. This is called ammonia volatilization
What causes ammonia volatilization?
The process of ammonia volatilization commonly takes place when nitrogen is in an organic form known as urea. Urea may originate from animal manure, urea fertilizers and, to a lesser degree, the decay of plant materials. Ammonia volatilization is most likely to take place when soils are moist and warm and the source of urea is on or near the soil surface. Ammonia volatilization will also take place on alkaline soils (pH greater than 8).
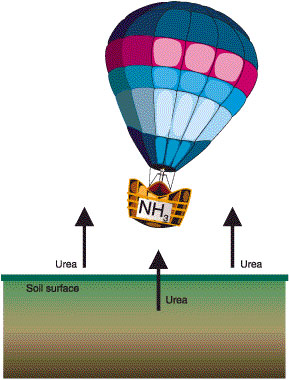 Figure 1
Figure 1
Urea is highly volatile. So if it is on or near the soil surface, it is easily converted and escapes as ammonia gas (NH3)
Preventing ammonia volatilization
Volatilization can be greatly reduced if manure and urea fertilizers are applied when soil and air temperatures are cool or when rain occurs soon after application. Physically mixing these materials into the soil shortly after application will also greatly reduce or prevent ammonia volatilization.
Impact on water quality
The process of ammonia volatilization does not directly impact water quality. Ammonia volatilization results in a net loss of nitrogen from the soil system. Therefore, indirectly it will result in less soil nitrogen being converted into the nitrate form. In this form, and when soils are excessively wet, nitrate is very mobile and easily moves with water. Nitrate is the form of nitrogen most likely to be lost to groundwater. For information involving health concerns regarding nitrates in groundwater, see MU publication WQ258.